Sterilizer and Autoclave Preventative Maintenance as Required by CDC, Joint Commission, and CMS
Sterilizer and Autoclave Preventative Maintenance as Required by CDC, Joint Commission, and CMS
Introduction
In the healthcare industry, ensuring the safety and efficacy of medical equipment is of utmost importance. Sterilizers and autoclaves play a critical role in preventing the spread of infections and maintaining a sterile environment in healthcare facilities. To ensure the proper functioning of these devices, it is essential to implement regular preventative maintenance measures. In this article, we will explore the importance of sterilizer and autoclave preventative maintenance as required by the Centers for Disease Control and Prevention (CDC), the Joint Commission, and the Centers for Medicare and Medicaid Services (CMS).
The Significance of Sterilizer and Autoclave Preventative Maintenance
Sterilizers and autoclaves are used to eliminate microorganisms from medical instruments, equipment, and supplies. They are vital in preventing the transmission of infections and ensuring patient safety. However, like any other equipment, sterilizers and autoclaves require regular maintenance to operate at their optimal level. Preventative maintenance not only helps identify and address potential issues before they become major problems but also extends the lifespan of the equipment and reduces the risk of equipment failure.
CDC Guidelines for Sterilizer and Autoclave Preventative Maintenance
The CDC provides guidelines for healthcare facilities to follow when it comes to sterilizer and autoclave preventative maintenance. These guidelines aim to ensure that these devices are functioning properly and effectively. Some key recommendations include:
- Regular Inspection: Healthcare facilities should conduct routine inspections of sterilizers and autoclaves to check for any visible signs of damage or wear. This includes inspecting seals, gaskets, filters, and other components for signs of deterioration.
- Cleaning and Decontamination: Sterilizers and autoclaves should be thoroughly cleaned and decontaminated according to manufacturer instructions. This helps remove any residue or buildup that may affect the sterilization process.
- Calibration and Testing: Regular calibration and testing of sterilizers and autoclaves are essential to ensure accurate temperature and pressure readings. This helps maintain the effectiveness of the sterilization process.
- Documentation: Proper documentation of all maintenance activities is crucial. This includes recording inspection results, cleaning and decontamination procedures, calibration and testing records, and any repairs or replacements performed.
By adhering to these guidelines, healthcare facilities can ensure that their sterilizers and autoclaves are operating as intended and meet the necessary standards for infection control.
Joint Commission Requirements for Sterilizer and Autoclave Preventative Maintenance
The Joint Commission, an independent, nonprofit organization that accredits and certifies healthcare organizations, also emphasizes the importance of sterilizer and autoclave preventative maintenance. As part of their accreditation process, the Joint Commission requires healthcare facilities to have a comprehensive maintenance program in place for all critical equipment, including sterilizers and autoclaves.
Key requirements set forth by the Joint Commission include:
- Written Maintenance Program: Healthcare facilities must have a written maintenance program that outlines the specific procedures and schedules for sterilizer and autoclave maintenance. This program should include regular inspections, cleaning protocols, calibration procedures, and documentation practices.
- Staff Training: Healthcare facilities must ensure that staff members responsible for sterilizer and autoclave maintenance are properly trained and competent in performing the necessary maintenance tasks. Training should cover equipment operation, maintenance procedures, and safety protocols.
- Equipment Performance Monitoring: Regular monitoring of sterilizer and autoclave performance is essential to identify any deviations from expected standards. This may involve monitoring temperature and pressure readings, as well as conducting biological indicator tests to verify the effectiveness of the sterilization process.
- Documentation and Record-Keeping: Similar to the CDC guidelines, the Joint Commission requires healthcare facilities to maintain detailed records of all sterilizer and autoclave maintenance activities. This includes inspection reports, cleaning and calibration records, staff training documentation, and any repairs or replacements performed.
By meeting the requirements set forth by the Joint Commission, healthcare facilities can demonstrate their commitment to maintaining high standards of equipment maintenance and patient safety.
CMS Regulations for Sterilizer and Autoclave Preventative Maintenance
The Centers for Medicare and Medicaid Services (CMS) also play a role in ensuring the proper maintenance of sterilizers and autoclaves in healthcare facilities. CMS regulations require healthcare facilities to meet certain standards to participate in Medicare and Medicaid programs.
Key CMS regulations related to sterilizer and autoclave preventative maintenance include:
- Condition of Participation: Healthcare facilities must comply with the CMS Conditions of Participation, which include requirements for equipment maintenance. This ensures that healthcare facilities maintain a safe and effective environment for patient care.
- Survey and Certification Process: CMS conducts periodic surveys and certifications to assess healthcare facilities’ compliance with regulations. During these surveys, the maintenance and performance of sterilizers and autoclaves are evaluated to ensure they meet the necessary standards.
- QualityAssurance and Performance Improvement: CMS encourages healthcare facilities to implement quality assurance and performance improvement programs that include regular monitoring and evaluation of sterilizer and autoclave maintenance. This helps identify areas for improvement and ensures ongoing compliance with CMS regulations.
- Prompt Response to Deficiencies: In the event that deficiencies are identified during CMS surveys or inspections, healthcare facilities must take prompt action to address and correct these issues. This may involve implementing corrective measures, conducting additional training, or seeking necessary repairs or replacements.
By adhering to CMS regulations, healthcare facilities can maintain their eligibility to participate in Medicare and Medicaid programs and demonstrate their commitment to providing safe and effective patient care.
Frequently Asked Questions (FAQ)
1. Why is preventative maintenance important for sterilizers and autoclaves?
Preventative maintenance is important for sterilizers and autoclaves to ensure their proper functioning, prevent equipment failure, and maintain a sterile environment in healthcare facilities. Regular maintenance helps identify and address potential issues before they become major problems, extends the lifespan of the equipment, and reduces the risk of infections.
2. How often should sterilizers and autoclaves be inspected?
Sterilizers and autoclaves should be inspected regularly, as recommended by the manufacturer and in accordance with CDC guidelines. This may vary depending on the specific model and usage, but typically inspections are conducted on a monthly or quarterly basis.
3. What are the consequences of not performing preventative maintenance on sterilizers and autoclaves?
Failure to perform preventative maintenance on sterilizers and autoclaves can lead to equipment malfunction, compromised sterilization effectiveness, increased risk of infections, and potential non-compliance with regulatory requirements. It can also result in costly repairs or the need for premature equipment replacement.
4. Can healthcare facilities perform sterilizer and autoclave maintenance in-house, or should they hire external service providers?
Healthcare facilities can choose to perform sterilizer and autoclave maintenance in-house if they have the necessary expertise and resources. However, it is important to ensure that staff members responsible for maintenance are properly trained and competent. Alternatively, healthcare facilities can hire external service providers who specialize in equipment maintenance to ensure compliance with guidelines and regulations.
5. How can healthcare facilities ensure proper documentation of sterilizer and autoclave maintenance?
Healthcare facilities can ensure proper documentation of sterilizer and autoclave maintenance by implementing a structured record-keeping system. This includes maintaining inspection reports, cleaning and calibration records, staff training documentation, and records of any repairs or replacements performed. Electronic systems or software specifically designed for equipment maintenance can help streamline the documentation process.
Contact Auxo Medical for Trusted Autoclave and Sterilizer Preventative Maintenance
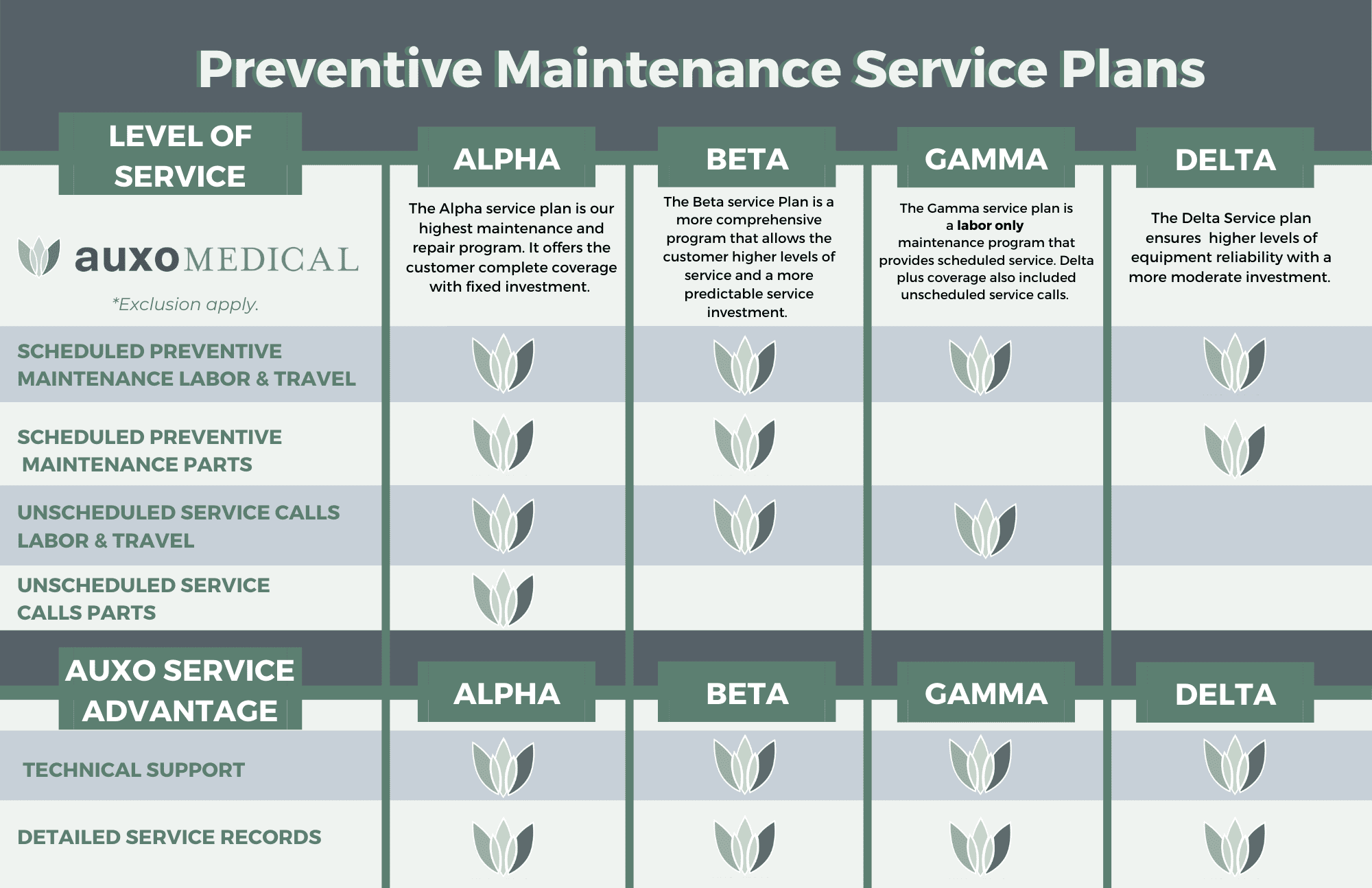 When it comes to ensuring the reliability and efficacy of your autoclaves and sterilizers, look no further than Auxo Medical for trusted preventative maintenance services. As a leading name in the industry, Auxo Medical is committed to providing comprehensive maintenance solutions that keep your essential equipment in top working condition and up to regulation standards. Regular servicing by our skilled technicians helps prevent unexpected breakdowns, extends the life of your machinery, and ensures compliance with stringent health and safety standards. Auxo Medical’s preventative maintenance plans are meticulously designed to address all aspects of sterilizer performance, from routine inspections to timely repairs, ensuring your operations run smoothly and efficiently. With Auxo Medical, you can have peace of mind knowing that your sterilization processes are in expert hands. Contact Auxo Medical today by filling out a service request online or call our office at (888) 728-8448.
When it comes to ensuring the reliability and efficacy of your autoclaves and sterilizers, look no further than Auxo Medical for trusted preventative maintenance services. As a leading name in the industry, Auxo Medical is committed to providing comprehensive maintenance solutions that keep your essential equipment in top working condition and up to regulation standards. Regular servicing by our skilled technicians helps prevent unexpected breakdowns, extends the life of your machinery, and ensures compliance with stringent health and safety standards. Auxo Medical’s preventative maintenance plans are meticulously designed to address all aspects of sterilizer performance, from routine inspections to timely repairs, ensuring your operations run smoothly and efficiently. With Auxo Medical, you can have peace of mind knowing that your sterilization processes are in expert hands. Contact Auxo Medical today by filling out a service request online or call our office at (888) 728-8448.


