A Comprehensive Guide to Steris Steam Sterilizers
In the world of sterilization, Steris steam sterilizers stand as a pinnacle of excellence. With their cutting-edge technology and unmatched performance, these robust machines ensure the highest level of cleanliness and safety in various industries, ranging from healthcare to pharmaceuticals. A comprehensive understanding of Steris steam sterilizers is essential for anyone seeking to make an informed decision about their sterilization needs.
Featuring state-of-the-art engineering and innovative designs, Steris steam sterilizers offer a wide range of features that cater to diverse requirements. From small-scale tabletop models ideal for clinics and laboratories to large-scale floor-standing units suitable for bustling hospitals or research facilities, Steris provides an extensive selection designed with precision and reliability in mind.
Choosing the Right Steris Sterilizer Model for Your Specific Needs
If you are in the process of choosing a Steris sterilizer model, it is essential to have a clear understanding of your specific needs and select a machine that seamlessly matches them. With numerous options to choose from in the market, it can be overwhelming to make the correct decision. But worry not, as this thorough guide will provide you with the necessary information and understanding to make a well-informed choice.
In order to choose the ideal Steris sterilizer model for your specific needs, start by evaluating the size and capacity required for your facility. Consider factors such as the volume of instruments or equipment you need to sterilize on a regular basis, as well as any expected growth or changes in demand. It is essential to select a model that can accommodate your current workload efficiently while leaving room for future expansion.
The Importance of a Quality Steris Sterilizer
When it comes to the critical task of sterilization, compromising on quality is simply not an option. A high-quality Steris sterilizer is the foundation of any successful sterilization process, ensuring that your instruments and equipment are effectively decontaminated to maintain a safe and hygienic environment.
A reliable Steris sterilizer provides peace of mind, knowing that all microorganisms, including bacteria, viruses, and spores, are eradicated. This is particularly crucial in healthcare facilities where patient safety is paramount. By investing in a quality Steris sterilizer, you can trust that every cycle yields consistent results, minimizing the risk of contamination and preventing potentially harmful infections.
Understanding Your Unique Sterilization Requirements
It is crucial to have a comprehensive understanding of your specific sterilization needs in order to choose the ideal Steris sterilizer model. Every facility or organization possesses a distinctive assortment of sensitive instruments, materials, or equipment that necessitates precise sterilization for the sake of safety and efficacy.
Consider the nature of the items you need to sterilize: Are they heat-sensitive? Do they require a specific temperature range during the sterilization process? Is there a need for compatibility with certain materials? By closely examining these factors, you can determine whether a low-temperature Steris sterilizer or a high-temperature one would be more suitable for your organization. Understanding these intricacies will guide you in making an informed decision and empower you to confidently choose the most appropriate Steris sterilizer model.
Evaluating the Size and Capacity of Steris Sterilizer Models
When selecting the appropriate Steris sterilizer model, it is vital to take into account its size and capacity. Examining your precise requirements in terms of load size and volume is critical to guarantee the best sterilization outcomes. Although larger models may appear enticing as they can accommodate more items, it is crucial to find a balance between capacity and efficiency.
When considering your facility or practice, whether it is a small clinic or a large hospital, it is important to evaluate the volume of instruments that need to be sterilized on a daily basis. This assessment will help determine if a smaller tabletop model or a larger floor-standing unit is more appropriate. It is also crucial to consider any projected future growth for your facility. Opting for a slightly bigger sterilizer that allows for expansion can prevent any regrets in the future.
Considering the Sterilization Cycle Time and Efficiency
When it comes to choosing the right Steris sterilizer model for your specific needs, one crucial factor to consider is the sterilization cycle time and efficiency. The time it takes for a sterilizer to complete a cycle is not only a matter of convenience but also impacts productivity and patient care.
An efficient sterilizer can significantly enhance workflow in a busy healthcare facility, allowing for more efficient use of time and resources. Shorter cycle times mean faster turnaround for instruments, ensuring that they are readily available whenever needed. This reduces waiting times for patients and enables healthcare professionals to operate smoothly without unnecessary delays.
However, it’s important to note that while shorter cycle times may be desirable, efficiency should not be compromised. A balance must be struck between speed and thoroughness of the sterilization process. Opting for a Steris sterilizer model that provides both rapid cycles and reliable effectiveness ensures that your instruments are not only cleaned quickly but also thoroughly disinfected, leaving you with peace of mind.
The Significance of Sterilization Validation and Compliance
Sterilization validation and compliance are vital aspects of ensuring the safety and efficacy of any Steris sterilizer model. The process of sterilization validation involves verifying that the chosen model consistently achieves the desired level of microbial reduction, thus eliminating potential pathogens that may compromise patient safety. Compliance with industry standards and regulations is equally important, as it reflects a commitment to maintaining high-quality standards in healthcare facilities.
By adhering to strict sterilization validation protocols, healthcare professionals can have confidence in the effectiveness of their chosen Steris sterilizer model. This assurance allows for the smooth operation of medical facilities, enhancing patient care while reducing the risk of infection transmission. Furthermore, compliance with regulatory requirements not only ensures patient safety but also demonstrates an organization’s dedication to ethical practices and continuous improvement.
New or Refurbished? Exploring the Options
When it comes to purchasing a Steris sterilizer, one important decision to make is whether to opt for a new or refurbished model. Both options have their merits, and understanding the nuances can help you make an informed choice that aligns with your specific needs.
Investing in a brand-new Steris sterilizer guarantees the latest advancements in technology, ensuring optimal performance and reliability. With cutting-edge features and improved efficiency, a new model offers peace of mind and the satisfaction of being at the forefront of sterilization innovation. Additionally, new models often come with warranties that provide added protection and reassurance.
A refurbished Steris Sterilizer offers numerous benefits for medical facilities and professionals. Firstly, opting for a refurbished sterilizer can significantly reduce costs compared to purchasing a brand new unit. This is especially advantageous for small or budget-conscious healthcare institutions that still require a reliable and robust sterilizer. Additionally, refurbished Steris sterilizers are thoroughly inspected, cleaned, and serviced by experts to ensure they meet the necessary safety and performance standards. This means that despite being used, a refurbished sterilizer can function just as effectively as a new one. Moreover, by choosing a refurbished sterilizer, medical facilities can contribute to sustainability efforts by reducing the demand for new manufacturing and lowering the overall carbon footprint in the healthcare industry. Overall, the benefits of a refurbished Steris Sterilizer include cost savings, reliable performance, and eco-friendliness, making it a compelling choice for medical facilities in need of a sterilizer.
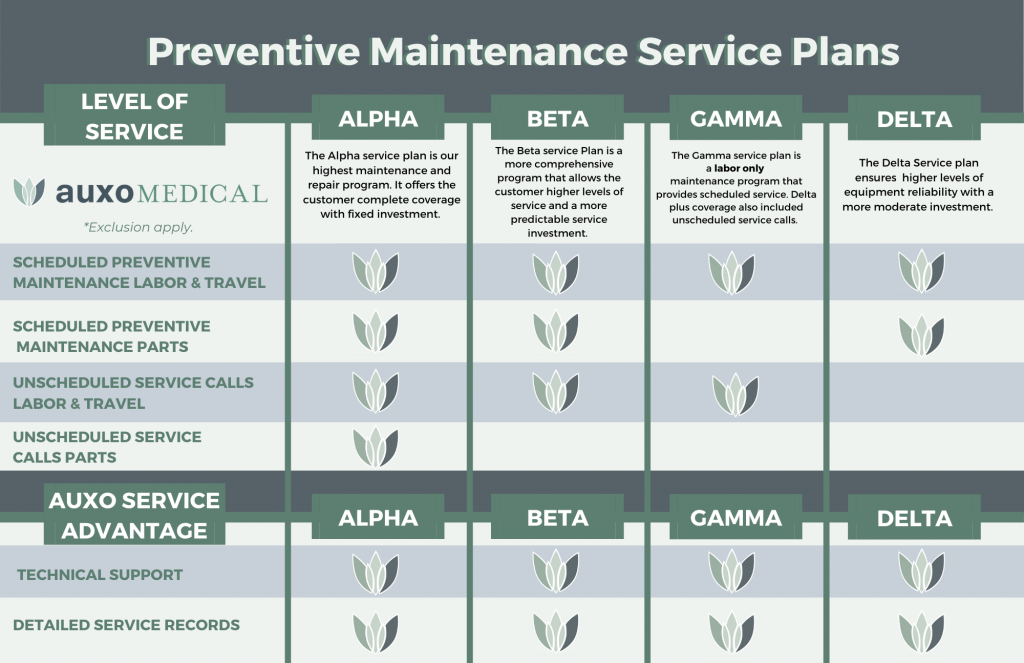
Purchasing and Installation of Your Steris Sterilizer through Auxo Medical
When it comes to purchasing and installing a Steris sterilizer, Auxo Medical is the ideal partner to trust. With our expertise and experience in the medical equipment industry, we ensure a seamless and hassle-free process for our customers. Auxo Medical offers a wide range of Steris sterilizers to choose from, catering to the specific needs of healthcare facilities. our team of knowledgeable professionals guides customers through the selection process, providing valuable insights and recommendations. Moreover, Auxo Medical takes care of the entire installation process, ensuring that the sterilizer is set up correctly and efficiently. With our commitment to customer satisfaction, Auxo Medical guarantees a smooth purchasing and installation experience for all Steris sterilizer users.
Steris Sterilizer Maintenance and Repair through Auxo Medical
Ensuring the smooth operation of your Steris sterilizer is essential for maintaining a safe and efficient sterilization process. With Auxo Medical’s expert maintenance and repair services, you can trust that your sterilizer will be in capable hands. Our highly trained technicians have a deep understanding of Steris sterilizers, allowing them to diagnose and resolve any issues swiftly.
At Auxo Medical, we believe in proactive maintenance to minimize the risk of breakdowns and maximize the lifespan of your equipment. Our comprehensive maintenance program includes regular inspections, cleaning, and calibration to guarantee optimal performance. In the event that your sterilizer requires repairs, our technicians are equipped with state-of-the-art tools and genuine Steris replacement parts to ensure effective solutions.
Now that you have delved into the intricacies of choosing the right Steris sterilizer model, it’s time to take action and acquire the perfect equipment for your needs. When it comes to purchasing a new or refurbished Steris sterilizer, there is no better partner than Auxo Medical. With our extensive knowledge and commitment to providing top-notch products and services, we ensure a seamless experience from start to finish.
At Auxo Medical, we understand that investing in a sterilizer is a significant decision for any healthcare facility or laboratory. That is why we offer an array of new and refurbished Steris sterilizers, providing you with options tailored to your budget and requirements. Our team of experts will guide you through the selection process, taking into account factors such as size, capacity, cycle time, and validation needs. Whether you opt for a brand-new model or choose from our carefully inspected refurbished units, rest assured that every sterilizer meets the highest quality standards. View our online catalogue of Steris sterilizers or call our Steris sterilizer experts at Auxo Medical toll-free at (888) 728-8448 for a customized quote .
 Hydrogen Peroxide Sterilizers are a type of sterilizer used to kill bacteria, viruses, fungi, and other microorganisms in healthcare settings. This sterilization method is widely used in hospitals, laboratories, and other medical facilities. It is a safe, effective, and economical way to sterilize medical instruments and surfaces. They use hydrogen peroxide to kill microorganisms. This chemical is highly reactive and breaks down quickly into oxygen and water, leaving no residue behind. It is also non-toxic and safe for humans and the environment.
Hydrogen Peroxide Sterilizers are a type of sterilizer used to kill bacteria, viruses, fungi, and other microorganisms in healthcare settings. This sterilization method is widely used in hospitals, laboratories, and other medical facilities. It is a safe, effective, and economical way to sterilize medical instruments and surfaces. They use hydrogen peroxide to kill microorganisms. This chemical is highly reactive and breaks down quickly into oxygen and water, leaving no residue behind. It is also non-toxic and safe for humans and the environment.
 Medical and dental professionals understand the importance of keeping their instruments and other equipment clean and sanitized. To ensure that their instruments are properly disinfected, they rely on washer-disinfector detergents. These detergents are specially formulated to kill harmful bacteria and other microbes that can spread disease.
Medical and dental professionals understand the importance of keeping their instruments and other equipment clean and sanitized. To ensure that their instruments are properly disinfected, they rely on washer-disinfector detergents. These detergents are specially formulated to kill harmful bacteria and other microbes that can spread disease. Are you in need of washer-disinfector equipment, detergents and supplies for your medical facility? Look no further than Auxo Medical. With a wide selection of washer-disinfector equipment, detergents and supplies, we provide the best quality products at the most competitive prices.
Are you in need of washer-disinfector equipment, detergents and supplies for your medical facility? Look no further than Auxo Medical. With a wide selection of washer-disinfector equipment, detergents and supplies, we provide the best quality products at the most competitive prices.