Midmark 230 Power Procedure Chair Available at Auxo Medical
Available at Auxo Medical, the Midmark 230 Power Procedure Chair is an adaptable and dependable choice for healthcare facilities in need of a high-quality chair for various medical procedures. It combines advanced features and an ergonomic design to provide exceptional comfort for both patients and professionals. Below is an overview of its key features, specifications, and customizable options.
Key Features
- Rotational Base
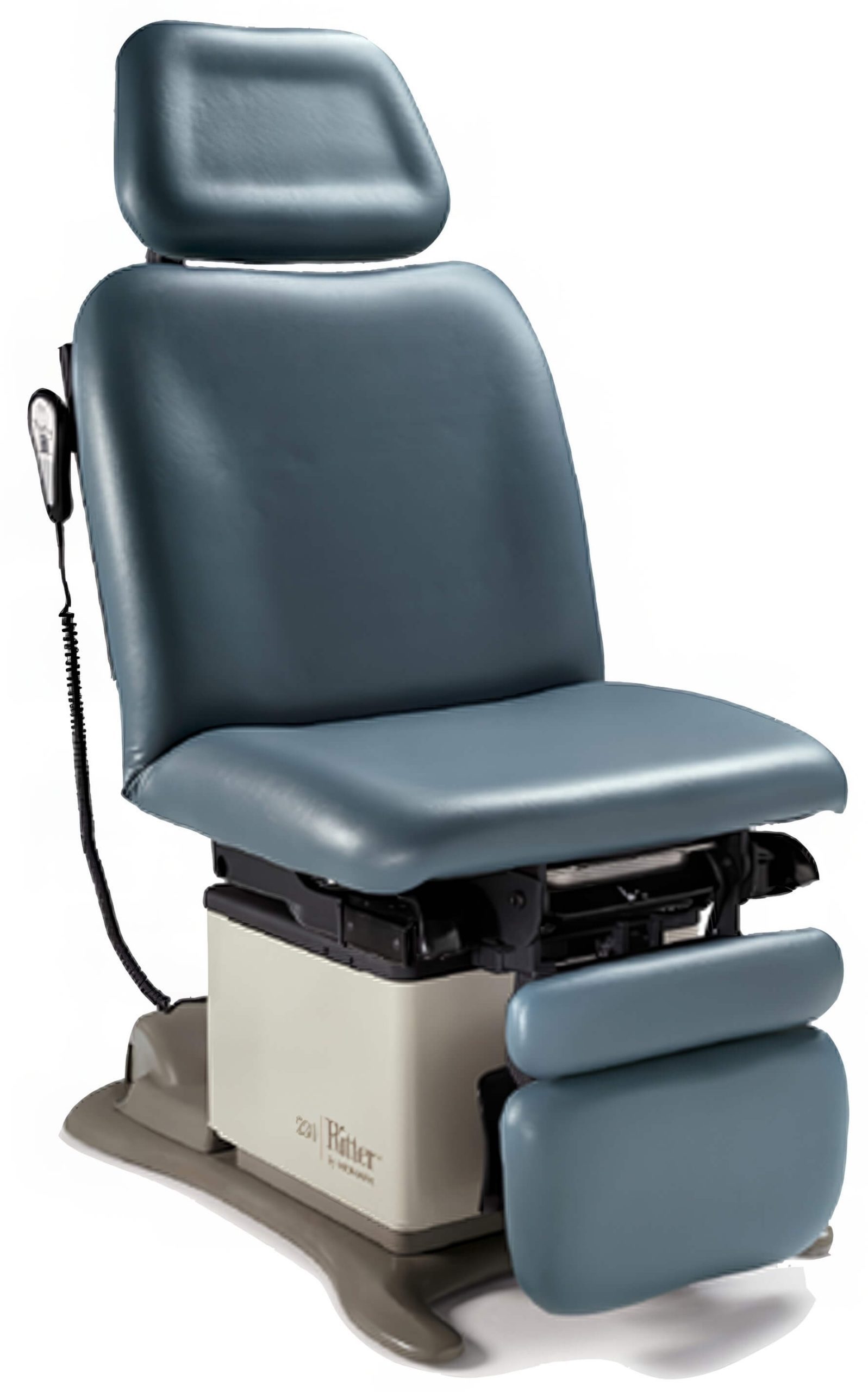 The chair has a rotational base that rotates 180 degrees, allowing healthcare professionals to access patients easily without needing to reposition the chair.
The chair has a rotational base that rotates 180 degrees, allowing healthcare professionals to access patients easily without needing to reposition the chair. - Power Height Adjustment
With the power height adjustment mechanism, staff can effortlessly change the chair’s height to their preferred level, enhancing patient comfort and streamlining workflow during procedures. - Trendelenburg and Reverse Trendelenburg Positions
The chair supports both Trendelenburg and Reverse Trendelenburg positions, enabling optimal patient positioning for specific medical tasks, which improves safety and comfort. - Programmable Chair Positions
Healthcare professionals can save and recall preferred chair positions at the touch of a button, facilitating quick adjustments and boosting overall efficiency. - Ergonomic Design
Designed for patient comfort, the chair features a contoured seat and backrest, adjustable armrests, and optimal support to minimize discomfort during longer procedures. - Easy-to-Clean Surfaces
Constructed with easy-to-clean surfaces, the upholstery is resistant to fluids, stains, and damage, ensuring durability and hygiene. - Safety Features
The Midmark 230 includes essential safety features like an adjustable patient restraint system, foot control lockout, and an emergency stop button for added peace of mind.
Specifications
- Weight Capacity: Up to 450 lbs (204 kg)
- Seat Width: 27 inches (68.6 cm)
- Overall Dimensions: 74.5 inches (189.2 cm) long, 27 inches (68.6 cm) wide, 21.5 inches (54.6 cm) high
- Power Requirements: Operates on standard electrical power, with voltage options to fit various facility needs.
Customizable Options
The Midmark 230 Power Procedure Chair offers several options to tailor it to your facility’s requirements:
- Upholstery Colors: Choose from a variety of colors to match your facility’s aesthetic.
- Arm Styles: Options include pivoting and adjustable swing-away armrests for flexibility.
- Accessories: Add-ons such as paper roll holders, IV poles, and procedure lights enhance functionality.
Purchase the Midmark 230 Power Procedure Chair From Auxo Medical
The Midmark 230 Power Procedure Chair, available to purchase from Auxo Medical, is a versatile solution for healthcare facilities seeking quality and comfort in medical procedures. Its advanced features, ergonomic design, and customizable options make it an ideal choice for enhancing procedural capabilities.
Midmark 230 Power Procedure Chair
Request Quote
Extending the stirrups and treatment pan, lowering the foot support and tilting the seat provide the perfect position for OB/GYN exams and procedures.
Description
Extending the stirrups and treatment pan, lowering the foot support and tilting the seat provide the perfect position for OB/GYN exams and procedures.
The table supports exams and procedures requiring supine, prone, left lateral or right lateral positioning.
Tool-less reconfigure of the foot support provides the perfect position for colon and rectal exams and procedures.
Whether better positioning the patient, treating for shock or faintness or increasing anesthesia onset, the Trendelenburg position offers a full 30° of tilt.
A 24-inch wide and flat upholstery option offers greater accessibility to the patient. The seamless design ensures a great look and easy-to-clean surface.
Articulating arm board accessory supports patient’s arm. Its ball joint design permits multi-directional movement.
Locking casters accessory provides mobility without sacrificing valuable floor space.
Options
Electrical Outlets: Duplex, hospital grade*
Rotation: Models equipped with rotation option add 3 1/2 inches to minimum and maximum height specifications
Flat upholstery set, 24 inches wide
Patient Load Rating: 450 lbs. (204 kg); Stirrups are standard
Height: Minimum 22.5 inches (57.2 cm); Maximum 40 inches (101.6 cm)
Seat Tilt Angle Range: 0° to 30°
Back Support Angle Range: 0° to 85°
Foot Support Angle Range: 0° to 90°
Upholstery Width Dimensions:
Headrest: 18 inches (45.7 cm)
Back support: 27 inches (68.6 cm)
Seat: 27 inches (68.6 cm)
Foot support: 18 inches (45.7 cm)
Patient Support Surface Length:
Flat position: 72 inches (182.9 cm)
Flat position with headrest extended: 83 inches (210.8 cm)
Paper Roll Holder Capacity: Holds 18 × 3.5 inch paper roll (45.7 × 8.9 cm)
Stainless Steel Treatment Pan Dimensions: 11.5 (H) × 9 (W) × 2.5 (D) inches (29.2 × 22.9 × 6.4 cm)
Optional Electrical Outlet Rating: 115 VAC, 3 Amps
Additional information
| Condition |
|---|

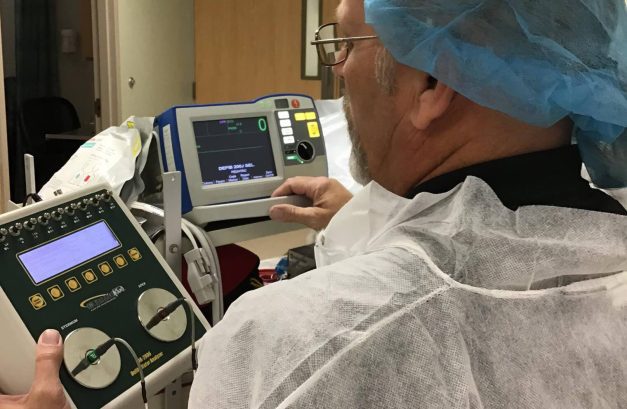

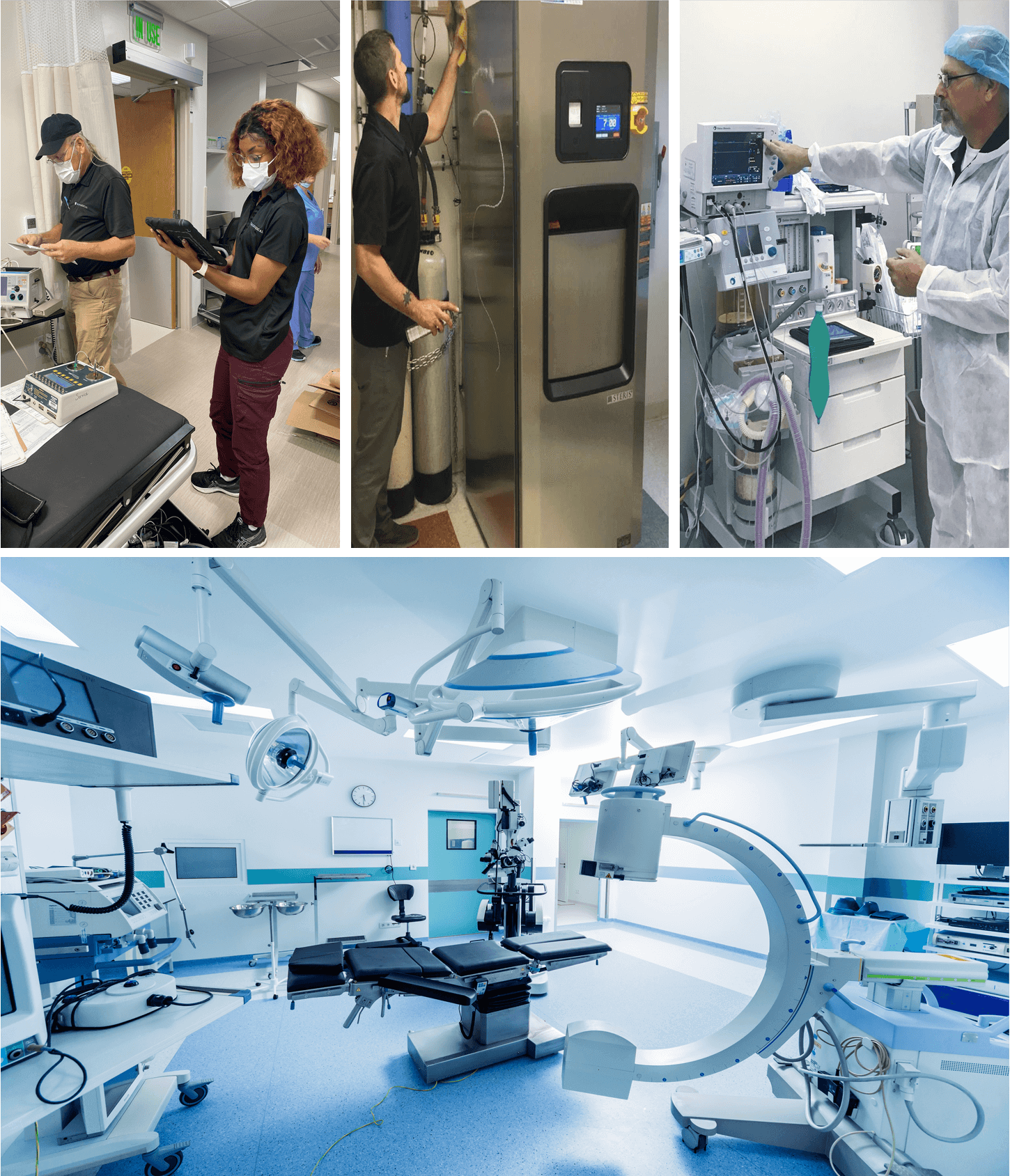 At Auxo Medical, we provide comprehensive services to help you assess and extend the lifespan of your medical equipment. Here’s how we can support your facility:
At Auxo Medical, we provide comprehensive services to help you assess and extend the lifespan of your medical equipment. Here’s how we can support your facility:
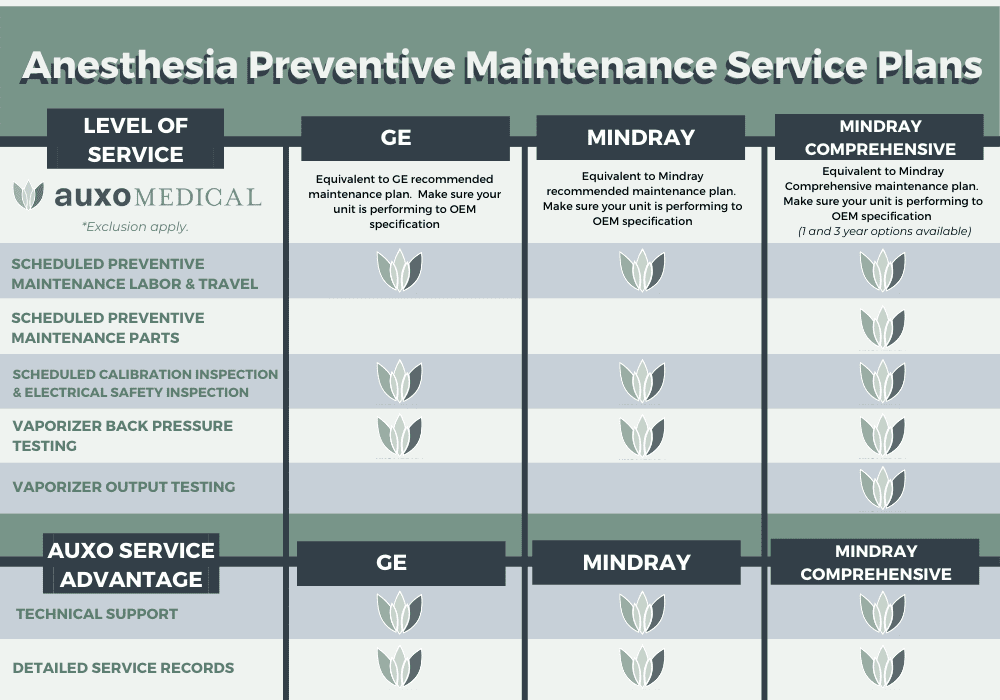 Preventative Maintenance Plans
Preventative Maintenance Plans






 Specialized Services Across Various Equipment Categories
Specialized Services Across Various Equipment Categories




